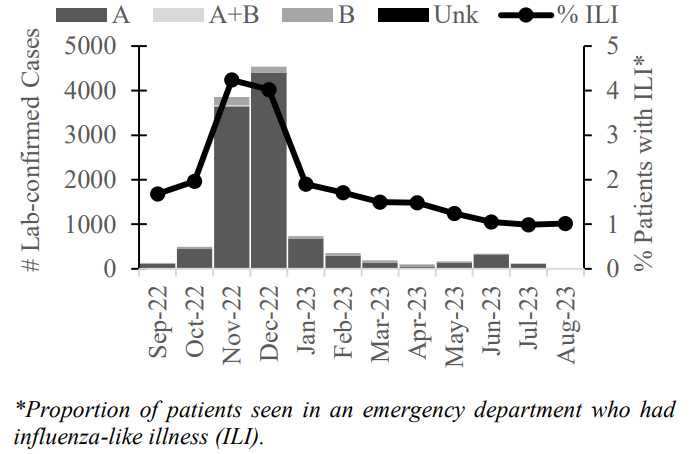
Multiple infections with variant* influenza A (H3N2v) viruses have been identified in 3 states in recent weeks. From July 12 through August 3, 2012, 16 cases of H3N2v were reported and confirmed by CDC. This virus was first detected in humans in July 2011. It has also been isolated in U.S. swine in many U.S. states.
Since July 12, 2011, there have been 29 cases of H3N2v virus infection, including the 16 cases occurring in the last three weeks. All 29 cases were infected with H3N2v viruses that contain the matrix (M) gene from the influenza A (H1N1)pdm09 virus. This M gene may confer increased transmissibility to and among humans, compared to other variant influenza viruses. All cases have been laboratory-confirmed at CDC. Each of the 16 cases identified since July 12, 2012, reported contact with swine prior to illness onset; in 15 cases, contact occurred while attending or exhibiting swine at an agricultural fair.
While the viruses identified in these cases are genetically nearly identical, separate swine exposure events in each state were associated with human infections. There is no indication that the cases in different states are epidemiologically related.
|
|
Clinical characteristics of the 16 H3N2v recent cases have been generally consistent with signs and symptoms of seasonal influenza, and have included fever, cough, pharyngitis, myalgia, and headache. No hospitalizations or deaths have occurred among the 16 confirmed cases since July 2012. Public health and agriculture officials are investigating the extent of disease among humans and swine, and additional cases are likely to be identified as the investigation continues.
Novel influenza A virus infection has been a nationally notifiable condition in the United States since 2007. Since that time, human infection with animal-origin influenza viruses has been rare, with ≤6 cases reported each year, until 2011 when 14 cases were identified. While most of the cases are thought to have been infected as a result of close contact with swine, limited human-to-human transmission of this virus was identified in some cases in 2011. Therefore, enhanced influenza surveillance is indicated, especially in regions and states with confirmed H3N2v cases.
|
|
Interim Recommendations for the Public
-
Persons who are at high risk for influenza complications (e.g., underlying chronic medical conditions such as asthma, diabetes, heart disease, or neurological conditions, or who are pregnant or younger than 5 years, older than 65 years of age or have weakened immune systems) should consider avoiding exposure to pigs and swine barns this summer, especially if ill pigs have been identified.
-
Persons engaging in activities that may involve swine contact, such as attending agricultural events or exhibiting swine, should wash their hands frequently with soap and running water before and after exposure to animals; avoid eating or drinking in animal areas; and avoid close contact with animals that look or act ill.
-
Patients who experience influenza-like symptoms following direct or close contact with pigs and who seek medical care should inform their health care provider about the exposure.
-
Patients with influenza-like illness who are at high risk for influenza complications (e.g., underlying chronic medical conditions such as asthma, diabetes, heart disease, or neurological conditions, or who are pregnant or younger than 5 years, older than 65 years of age or have weakened immune systems) should see their health care provider promptly to determine if treatment with antiviral medications is warranted.
-
Influenza viruses have not been shown to be transmissible to people through eating properly handled and prepared pork or other products derived from pigs. For more information about the proper handling and preparation of pork, visit the USDA website fact sheet “Fresh Pork from the Table.”
|
|
Interim Recommendations for Health Care Providers
-
Clinicians who suspect influenza in persons with recent exposure to swine should obtain a nasopharyngeal swab or aspirate from the patient, place the swab or aspirate in viral transport medium, and contact their state or local health department to arrange transport and request a timely diagnosis at a state public health laboratory.
-
Reverse-transcription polymerase chain reaction (RT-PCR) testing for influenza should be considered for patients with influenza-like illness prior to the start of the traditional influenza season in October.
-
RT-PCR testing for influenza should be considered throughout the year for patients with influenza-like illness reporting recent swine exposure and for those who can be epidemiologically linked to confirmed cases of variant influenza.
-
Commercially available rapid influenza diagnostic tests (RIDTs) may not detect H3N2v virus in respiratory specimens. Therefore, a negative rapid influenza diagnostic test result does not exclude infection with H3N2v or any influenza virus. In addition, a positive test result for influenza A cannot confirm H3N2v virus infection because these tests cannot distinguish between influenza A virus subtypes (they do not differentiate between human influenza A viruses and H3N2v virus). Therefore, respiratory specimens should be collected and sent for RT-PCR testing at a state public health laboratory.
-
Clinicians should consider antiviral treatment with oral oseltamivir or inhaled zanamivir in patients with suspected or confirmed H3N2v virus infection. Antiviral treatment is most effective when started as soon as possible after influenza illness onset.
|
|
For more information:
- “Interim Guidance on Case Definitions to be Used for Investigations of Influenza A (H3N2) Variant Virus Cases” for state and local health departments is available at https://www.cdc.gov/flu/swineflu/case-definitions.htm.
- “Prevention Strategies for Seasonal and Influenza A(H3N2)v in Health Care Settings” is available at https://www.cdc.gov/flu/swineflu/prevention-strategies.htm.
- “Interim Guidance on Specimen Collection, Processing and Testing for Patients with Suspected Influenza A (H3N2) Variant Virus Infection” for public health professionals is available at https://www.cdc.gov/flu/swineflu/h3n2v-testing.htm, and
- “Interim Guidance for Influenza Surveillance: Additional Specimen Collection for Detection of Influenza A (H3N2) Variant Infections” for state and local health departments is available at https://www.cdc.gov/flu/swineflu/h3n2v-surveillance.htm.
- Compendium of Measures to Prevent Disease Associated with Animals in Public Settings, 2011 is available athttps://nasphv.org/documentsCompendiumAnimals.html
*Influenza viruses that circulate in swine are called swine influenza viruses when isolated from swine, but are called variant viruses when isolated from humans.
The Centers for Disease Control and Prevention (CDC) protects people’s health and safety by preventing and controlling diseases and injuries; enhances health decisions by providing credible information on critical health issues; and promotes healthy living through strong partnerships with local, national, and international organizations.