ANN ARBOR, Mich. — Living with pulmonary arterial hypertension is challenging, but the way patients and their doctors can treat the rare heart disease is changing after a worldwide study published in the Dec. 24 in the New England Journal of Medicine.
Targeting a well-known disease pathway that opens blood vessels to the lungs and improves heart function often requires patients to use infusions and injections for treatment.
Data from the largest study ever of pulmonary hypertension shows the oral medication Selexipag led to a 40 percent reduction in hospitalizations and worsening symptoms among patients with pulmonary hypertension.
Selexipag gained U.S. Food and Drug Administration approval this week, setting the course for the therapy to be available for patients in January.
“For more than two decades we’ve targeted the prostacyclin pathway to induce vasodilation in these blood vessels in the lung,” says senior study author Vallerie McLaughlin, M.D., director of the Pulmonary Hypertension Program at the University of Michigan Frankel Cardiovascular Center.
“But because of the cumbersome nature of treatment, patients would often wait until late stages to begin therapy. Having an oral medication to attack the disease pathway will be a major advancement because less ill patients will be willing to begin this therapy,” McLaughlin says.
Selexipag demonstrated effectiveness while its most frequent adverse events were headache, diarrhea, nausea, muscle pain and joint pain. The side effects were considered consistent with prostacyclin therapy.
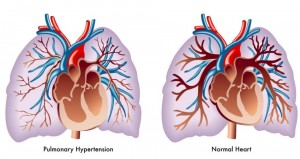
Pulmonary hypertension, which is high blood pressure in the loop of blood vessels connecting the heart and lungs, can make everyday activities exhausting. It often leads to life-threatening heart failure as the heart works harder to pump blood to the lungs.
The greatest number of cases is reported in women between ages 21 and 40 who most often experience fatigue and shortness of breath.
While a rare disease, research of causes and treatment of pulmonary hypertension is growing. The GRIPHON study is a standout because of its size and scope, enrolling 1,156 patients with pulmonary hypertension from 181 centers from 39 countries in North and South America, Europe, Asia-Pacific and Africa.
Collaborators from the U.S. and Europe led the GRIPON study, which was sponsored by Actelion Pharmaceuticals.
Source: UOFM [xyz-ihs snippet=”Adsense-responsive”]
