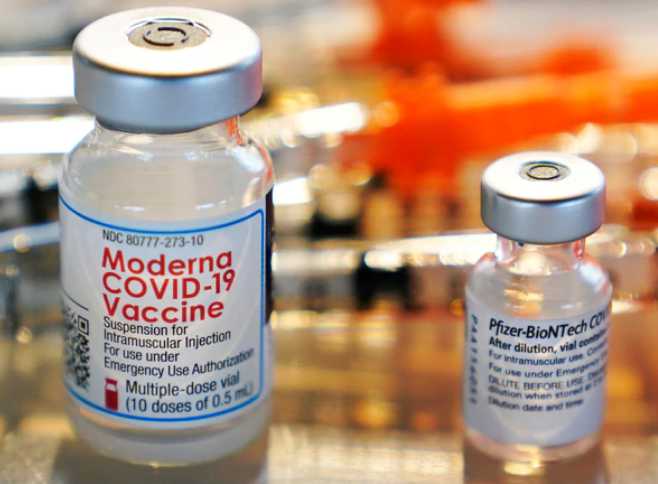
ANCHORAGE – The second COVID-19 vaccine authorized for emergency use by the U.S. Food and Drug Administration (FDA) arrived in Alaska on Monday. Distribution and administration of this vaccine, made by Moderna, will occur this week in Alaska alongside the continued roll-out of the Pfizer vaccine which started last Monday.
As of Sunday, 5,674 doses of Pfizer vaccine have been administered in Alaska and reported to Alaska’s VacTraAK immunization information system.
The Moderna vaccine received emergency use authorization from the FDA on Friday. The Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) recommended Saturday that Americans aged 18 and older receive the Moderna vaccine under emergency use. CDC Director Dr. Robert Redfield signed the ACIP recommendation, which began distribution of the Moderna vaccine on Sunday.
“We want to offer this vaccine to Alaskans as quickly as possible,” said Alaska’s Chief Medical Officer Dr. Anne Zink. “This is a major step in that direction. We’re extremely grateful for the hard work that has gone into developing this vaccine and ensuring its safety. Our role is to continue to distribute vaccine according to federal and state allocation plans to Alaskans who want it.”
According to current federal government estimates, Alaska will initially receive 26,800 doses of Moderna vaccine in addition to the 35,100 doses of the Pfizer vaccine, which began to be distributed and administered last week. These numbers include the Indian Health Service allocations for Alaska, but do not include vaccine allocated to Veterans Affairs and the Department of Defense.
The initial state allocations from Pfizer and Moderna include enough doses for 61,900 people. Both vaccines require a second dose; the second doses are being held in reserve by the federal government to ensure they will be available when needed. The follow-up dose of the Pfizer vaccine should be taken three weeks after the first dose while the separation between doses is four weeks for the Moderna vaccine.
The Pfizer and Moderna vaccines are similar in that they both use messenger RNA technology (mRNA) to protect people against the virus, they are highly effective, and they require two doses spaced apart. Both vaccines must be kept cold but the Moderna vaccine must be shipped at -4° Fahrenheit and is stable after thawing at refrigerator temperatures for 30 days and at room temperature for 12 hours. The Pfizer vaccine must be shipped at -94° Fahrenheit but can only be stored at refrigerated temperature for 5 days.
“The Moderna vaccine will help us reach more communities, especially those that have less access to cold storage,” said Tessa Walker Linderman, the DHSS co-lead of Alaska’s COVID-19 Vaccine Task Force. “Having both vaccines provides us more vaccine – and more flexibility.”
[content id=”79272″]
Who can get vaccinated, and when?
Vaccine availability remains limited at this time and will be allocated according to federal and state recommendations. ACIP provides recommendations about who should have the vaccine available to them first. States have the option to make further adjustments if needed. As per the Alaska Draft COVID-19 Vaccination Plan, the Alaska Department of Health and Social Services (DHSS) has convened the Alaska Vaccine Allocation Advisory Committee (AVAAC). The committee is comprised of Alaska clinicians, ethicists and other health professionals who review the ACIP guidance and scientific data and provide recommendations on the appropriate allocation of limited COVID-19 vaccine supplies in Alaska.
Over the course of several meetings, AVAAC reviewed ACIP recommendations for Phase 1a. The committee defined Phase 1a within three distinct tiers; individuals in the first two tiers are currently eligible to be vaccinated. The remaining Phase 1a tier is anticipated to be eligible Jan. 4, 2020.
Tier 1 of Phase 1a (currently being administered) includes:
- Hospital-based front-line health care workers who are frequently exposed to COVID-19 patients. For more details on who is included in this group, visit the Alaska Guidance for Allocating COVID-19 Vaccine information web page.
- Long-term care facility residents and staff. This includes skilled nursing facilities, assisted living homes, and Department of Corrections infirmaries providing assisted living care.
Tier 2 of Phase 1a (currently being administered) includes:
- Front-line EMS and fire service personnel providing medical services who are frequently exposed to COVID-19 patients and whose absence from work would compromise the ability of these critical medical services to continue. This tier includes personnel in certified ground-based and air medical services. This tier also includes community health aides and health workers providing EMS services.
- Community Health Aides/Practitioners
- Health care workers providing vaccinations to identified populations in Phase 1a
Tier 3 of Phase 1a (expected to be administered starting as early as Jan. 4, 2020):
- Workers in other health care settings at highest risk of contracting COVID-19 who are essential to the health care infrastructure and who regularly provide health care services that cannot be postponed or provided remotely. These workers meet all of the following criteria:
- They have direct patient contact, or have direct contact with infectious materials from patients; and,
- They provide essential services in a health care setting that cannot be offered remotely or performed via telework; and,
- They provide a service in a health care setting that cannot be postponed without detrimental impact to the patient’s short-term or long-term health outcomes; and,
- They need to be licensed and certified – this includes direct support professionals who provide personal care or home- and community-based services, laboratory technicians, phlebotomists, and workers performing COVID-19 testing.
On Dec. 20, ACIP issued further recommendations for Phases 1b and 1c vaccine allocation. Balancing between two high-risk groups, ACIP recommended that people aged 75 and older and front-line essential workers such as emergency responders, teachers and grocery store employees be next in line for the vaccine.
In Alaska, a public comment meeting for Phase 1b will occur Dec. 28, 2020. More information about the meeting, and how to provide comment, is available at the Alaska Guidance for Allocating COVID-19 Vaccine information web page.
[content id=”79272″]
DHSS prioritizes safety
The Alaska Department of Health and Social Services (DHSS) has been communicating with the CDC, FDA and health care partners in Alaska regarding the adverse reactions reported this week during COVID-19 vaccine clinics. Investigations into what might have caused the reactions are ongoing and DHSS is assisting.
“Safety is a top priority, and these systems are closely monitored by the CDC,” said Dr. Zink. “Reporting any adverse reaction is extremely important so we can continue to ensure the safety of these vaccines. All providers are encouraged to report all adverse reactions to the VAERS system so we can continue be as transparent as possible.”
As of today, DHSS is aware of 11 reports regarding possible allergic reactions from Alaska’s hospitals to CDC: Bartlett Regional Hospital (8), Providence Alaska (2) and Fairbanks Memorial Hospital (1). Two were identified as anaphylaxis and one of those resulted in hospitalization for ongoing monitoring. In the other three cases, symptoms were mild and not considered anaphylaxis. Symptoms have resolved in all cases and the hospitalized patient has been discharged and is doing well. The CDC said there appears to be no obvious geographic clustering of these reactions, nor was a specific production lot involved. People who experience anaphylaxis after the first dose should not receive a second dose, according to CDC recommendations.
In all of these situations, as recommended by the CDC and DHSS, symptoms were discovered during the 15-30 minute observation period following vaccination, and as required, the hospitals had medicine on hand to treat possible allergic reactions. Vaccinations are continuing as DHSS and its state and federal partners investigate these incidents and watch closely for any further allergic reactions.
“We strongly encourage anyone who experiences an adverse reaction to promptly report it to the CDC via the Vaccine Adverse Event Reporting System (VAERS),” said Alaska’s State Epidemiologist Dr. Joe McLaughlin. “Clinicians who will be administering COVID-19 vaccine should also review CDC’s guidance on anaphylaxis management to ensure that they are prepared to deal with allergic reactions should they occur.”
Vaccination providers administering a COVID-19 vaccine that is under Emergency Use Authorization are required by the Food and Drug Administration to report vaccine administration errors, serious adverse events, cases of Multisystem Inflammatory Syndrome, and cases of COVID-19 that result in hospitalization or death. Reporting is also encouraged for any other clinically significant adverse event, even if it is uncertain whether the vaccine caused the event.
For more information about VAERS visit: vaers.hhs.gov
For more information on COVID-19 vaccination in Alaska visit: covidvax.alaska.gov.
# # #