The Alaska Section of Epidemiology (SOE) conducts routine influenza surveillance throughout the year, with heightened surveillance occurring October through May. Influenza surveillance provides information on where influenza activity is happening, tracks influenza-related illness (ILI) and mortality and detects changes in the viral genome. Weekly surveillance reports are posted on the SOE influenza webpage.1 The purpose of this Bulletin is to provide an epidemiologic summary of the 2022–23 influenza season.
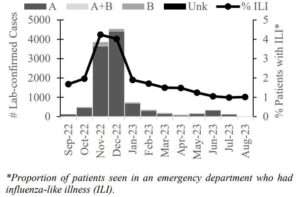
During the 2022–23 season in Alaska, influenza cases exceeded the counts of the previous four seasons beginning with an early spike in November/December 2022 (Figure). Influenza A dominated over B throughout the season (Figure). Seasonal influenza activity is difficult to predict. Surveillance is necessary for tracking influenza activity, monitoring circulating strains, and facilitating prompt response to outbreaks in high-risk settings.
Alaska implements a sentinel surveillance system for respiratory viruses annually, which attempts to strategically characterize specimens that represent Alaska’s vast geography and dispersed population. There were 2,757 specimens tested for influenza at the Alaska State Virology Laboratory (ASVL) during the 2022–23 respiratory season. The Centers for Disease Control and Prevention (CDC) received a subset (n=9) of those specimens for genome sequencing and antigenic typing, per specific CDC criteria. Another subset (n=3) of respiratory specimens were sent to New York – Wadsworth for pyrosequencing and antiviral resistance testing.
Influenza A/H3 was the predominating virus throughout this respiratory season. Further characterization determined that these viruses, as well as the influenza A/H1 viruses, were antigenically well matched to the vaccine component for the 2022–23 season. An exception to this was one influenza A/H3 strain (A/Darwin/6/2021-like LOW) that demonstrated lower affinity to antibodies produced by the vaccine in laboratory experiments. Influenza B viruses were also well matched to the vaccine. No antiviral resistant viruses were detected in Alaska’s submitted viruses.
Alaska clinicians play a crucial role in helping to characterize influenza activity statewide by performing rapid flu tests on patients in clinics and multiplex testing on patients in hospitals.
SOE participates in the United States Outpatient Influenza-like Illness Surveillance Network (ILINet). Data from participating outpatient providers in Alaska are pooled to create a statewide estimate for the weekly percentage of health care visits that are due to influenza-like illness (ILI). Patients presenting with a fever of ≥100ºF and a cough or sore throat are classified as having ILI. For more information on state and national ILINet data, see FluView Interactive. While ILI activity is less specific than laboratory-confirmed influenza, the two surveillance systems usually track well for trends.
During the 2022–23 season, five adult and no pediatric influenza-associated deaths were identified from clinician reports, hospitals, and Alaska death certificate reviews.
Based on VacTrAK data,4 influenza immunization coverage during the 2022–23 season was 24.2% among Alaskans aged 18+ years. This is similar to the prior season in Alaska but substantially lower than national estimates.
Compared to 2021–22, the 2022–23 influenza season saw a substantial increase in influenza activity, with an earlier start and peak relative to recent seasons. ILI activity was highest in November-December, with a similar pattern to influenza cases. Preliminary vaccine effectiveness against medically-attended influenza A(H3N2) for 2022–23 was estimated to be 45%.
1. Clinicians should urge all eligible patients aged >6 months to receive influenza vaccine annually by the end of October. Influenza vaccine is the most effective tool we have to prevent influenza-associated morbidity and mortality.
2. Clinicians can submit respiratory specimens from patients with ILI to ASVL for influenza testing; supplies can be obtained for free by calling 907-371-1000. Laboratory request forms are available at: https://health.alaska.gov/dph/Labs/Documents/publication s/Virologytestreq.pdf
3. Laboratories must report all positive influenza test results (including rapid test results) to SOE per 7 AAC 27.007. Laboratories are also encouraged to report the total number of tests performed and the number of positive results directly to CDC to help meet Alaska’s National Respiratory and Enteric Virus Surveillance System goals. 3 Call ASVL at 907-371-1000 for more information.
4. Health care providers must report suspected and confirmed influenza-associated deaths and unusual clusters of respiratory illness to SOE (call 907-269-8000 during business hours, or 800-478-0084 after hours).
[content id=”79272″]


